Hiperplasia Adrenal Congénita
Noviembre - 2014
The Child With Difficult to Control
Congenital Adrenal Hyperplasia
Peter C. Hindmarsh ; Clin Endocrinol. 2014;81(1):15-18.
The treatment of congenital adrenal hyperplasia requires administration of glucocorticoid and mineralocorticoid to replace the deficit resulting from the enzymatic block. During puberty, cortisol metabolism changes with an increase in clearance, which may lead to unstable control. This may require an increase in dosing frequency, and if this proves problematic, continuous subcutaneous hydrocortisone delivery using insulin pump technology can be valuable. The potential indications for such therapy along with some practical considerations are detailed in this Clinical Question report.
Clinical Scenario
A 14-year-old boy with congenital adrenal hyperplasia (CAH) was noted to have elevated plasma 17 hydroxyprogesterone (17OHP) and androstenedione concentrations over the previous 3 years. A standard replacement regimen of oral hydrocortisone of 12 mg/m2 body surface area/day given as three divided doses was used. Further increases in the frequency of the doses up to six times per day did not improve the biochemical parameters. He was in mid-puberty with genitalia stage 4 and pubic hair stage 4. Why should puberty be associated with problems with hydrocortisone dosing and what more can be done to the dosing regimen?
Physiology of Congenital Adrenal Hyperplasia During Puberty
Congenital adrenal hyperplasia is a disorder of adrenal steroid biosynthesis although some forms may also affect gonadal steroidogenesis. 21-hydroxylase, which catalyses the conversion of 17OHP to 11-deoxycortisol, is the most common enzyme deficiency. The mainstay of therapy is replacement of cortisol with a synthetic glucocorticoid, usually hydrocortisone. Over the years, a number of treatment regimens have been proposed. The most common is three times per day.[1] There has been a steady improvement in adult height over time as the dosing schedule has been reduced towards the normal cortisol production rate[2] although other outcomes remain poor.[3]There remains a question of whether dosing should be three or four times per day as the duration of hydrocortisone in the circulation is about 6 h. The use of longer acting preparations, such as prednisolone and dexamethasone, in both paediatric and adult practice has not met with success[3] and slow release preparations await development.[4]
In the case study, it is tempting to invoke poor concordance to explain the loss of control. This would at first sight seem inherently unlikely as some progress had been made in puberty which would be unlikely if control was that poor as androstenedione would have suppressed the hypothalamo-pituitary-gonadal axis. Careful assessment is required in situations such as this as cortisol metabolism changes during puberty. First, clearance is increased,[5] especially in females, through alterations in 11 beta-hydroxysteroid dehydrogenase activity leading to decreased reactivation to cortisol.[6] The half-life can be as low as 40 min in puberty compared to 80 min in pre- or postpuberty. Second, the increase in growth hormone production increases glomerular filtration. Third, oestradiol in both males and females increases cortisol binding globulin. The net effect is to reduce the circulating cortisol concentration, and if the fixed dosing of hydrocortisone is not changed, control of the hypothalamo-pituitary-adrenal axis is lost. This aspect of physiology can be assessed by undertaking a 24-h profile of plasma cortisol, which will allow an estimate to be made of cortisol peaks and troughs as well as duration of exposure to the hydrocortisone dose. This can be developed further with an IV study of hydrocortisone clearance, measurement of cortisol binding globulin and if necessary determination of the bioavailability of oral hydrocortisone.[7]
If clearance is rapid, then a more frequent regimen of hydrocortisone is required and if this does not restore good control then consideration should be given to delivering hydrocortisone using an insulin pump adapted for hydrocortisone delivery. This approach has met with success in individuals with Addison's disease[8] and congenital adrenal hyperplasia.[9]
Potential Indications for Hydrocortisone Pump Therapy
The use of pump therapy in CAH came about as a result of observing increased cortisol clearance in an individual.[9] To overcome the rapid clearance of hydrocortisone, a high frequency of oral hydrocortisone dosing was required, which was impractical and did not maintain adequate control. Introduction of hydrocortisone using an insulin pump normalized all the control measures of CAH, namely 17OHP and androstenedione, as well as delivering a physiological circadian rhythm of cortisol in the circulation. Since then, we have managed two other young people using this approach. One individual with CAH had developed severe gastritis from the use of prednisolone and the other was a young boy who was diagnosed with Addison's disease who also had rapid cortisol clearance. Table 1 outlines potential indications for hydrocortisone pump therapy.
Circadian Rhythm
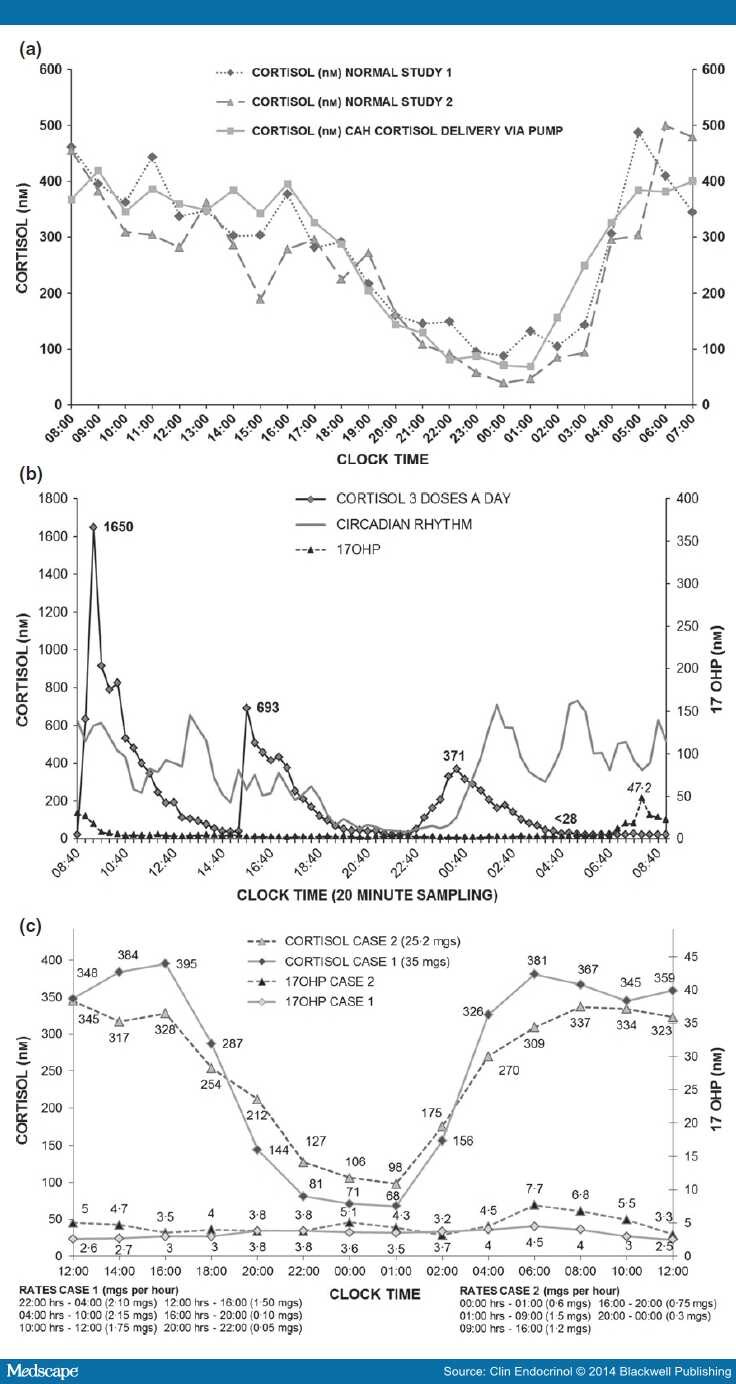
Plasma cortisol concentrations display a circadian rhythm (Fig. 1a). Following the morning peak, plasma cortisol concentrations remain between 150 and 300 nm for a considerable part of the day and early evening. Hydrocortisone dosing on a three times per day basis does not quite achieve this circadian rhythm (Fig. 1b) as there are periods of time where cortisol concentrations are either too high or too low. In the late evening particularly in children when they are going to sleep, cortisol concentrations would be at their maximum, if the tablet was given at bedtime, whereas in the early hours of the morning when high concentrations of cortisol are required to suppress, the ACTH drive cortisol concentrations are low. To a certain extent, this could be circumvented by giving the evening dose of hydrocortisone later at say 04.00 h.
Generally, these particular gaps in the therapeutic regimen do not cause major problems. It is only when individuals with rapid clearance rates have to receive hydrocortisone therapy that problems occur. In this situation, pump therapy is extremely valuable in delivering hydrocortisone in a physiological manner (Fig. 1a).

Figure 1.
(a) Upper panel. Comparison of 24 h Plasma cortisol concentrations between two healthy adult volunteers (diamond and triangle symbols with dashed lines) and patient with congenital adrenal hyperplasia on a continuous subcutaneous hydrocortisone infusion (squares and solid line). (b) Middle Panel. Plasma cortisol concentrations (diamonds) resulting from a three times per day oral dosing regimen of hydrocortisone with plasma 17-hydroxyprogesterone concentrations (triangles) illustrating escape from control in the early hours of the morning. (c). Lower Panel. 24 h plasma cortisol (diamonds Case 1 and open triangles Case 2) and 17-hydroxyprogesterone (open circles Case 1 and closed triangles Case 2) concentrations in two individuals with salt wasting congenital adrenal hyperplasia receiving continuous subcutaneous hydrocortisone infusions. Pump rates shown below.
Setting up the Pump – The Calculations
Before commencing hydrocortisone pump therapy, the clearance of cortisol in the individual should be determined as clearance plays an important role in the calculation of the delivery of hydrocortisone using the pump. Cortisol clearance can be calculated from an intravenous bolus injection of hydrocortisone.[10] An intravenous dose of 30 mg of hydrocortisone which generates a peak cortisol concentration about 5–10 min after administration of about 1500–1800 nm is sufficient.
Sampling should be every 5 min for the first 45 min and every 15 min thereafter until 90 min in order to obtain sufficient data to best describe the exponential decline of cortisol in the circulation. Clearance and half-life can be derived using classic pharmacological formulae.[10]
Once the clearance of cortisol is known, then the amount of hydrocortisone that needs to be delivered over a period of 1 h can be calculated using the formula:
To now deliver the hydrocortisone, we use the feature on the pump which is the basal infusion rate. On most pumps, this can be varied over the 24-h period, often at 30 min intervals. This is rarely required in adrenal insufficiency and longer infusion periods of 4–6 h are sufficient. Using the above-mentioned formula to work out, the concentrations of cortisol that are required generate a series of infusion rates which translate to the basal rates that need to be entered into the pump. The hydrocortisone is distributed in 6 hourly segments with 30% of the dose delivered between midnight and 06.00 h, 40% between 06.00 and 12.00 h, 20% between 12.00 and 18.00 and 10% between 18.00 and midnight.[11]Because the pump delivers the hydrocortisone over a 1-h period, it is necessary to always pre-empt the change by programming in the time of the change 1 h before it is required.
Using the basal rates also allows for temporary basal changes to be made. This means that the infusion of hydrocortisone can be increased up to 200% of what has been originally set. This is an immensely valuable function as periods of stress lasting more than 3–4 h e.g. illness can be coped with simply by changing the infusion rate. In addition, the user can also use the bolus function to give a subcutaneous bolus of hydrocortisone for brief stressful events lasting 3–4 h. Bolus injections of 3 mg of hydrocortisone are more than adequate to cover any stressful event. A further advantage is that the boluses can be repeated at regular intervals if the stressful condition persists. Line blockages are extremely rare and usually manifest as headache and nausea. They are dealt with by giving oral or IM dose of hydrocortisone, while the patient changes the cannula set. Hydrocortisone is extremely stable in the pump system, so set changes are only needed every 3 days.[12] Because of local skin reactions and abscess formation, we only recommend Solu-Cortef (Pfizer, Sandwich, UK) as the hydrocortisone preparation.
Cortisol Delivery on the Pump
Figure 1c shows the delivery of cortisol using the pump infusion system and the associated 17OHP measurements. Mimicking the circadian rhythm leads to plasma 17OHP concentrations within the normal range. When giving hydrocortisone orally, it has often been argued by clinicians that any 17OHP concentration within the normal range may represent over treatment. The pump delivery of hydrocortisone and the normalization of 17OHP which occurs at the same time as the delivery of plasma cortisol of normal concentration argues against this in this situation. Normalization of 17OHP concentrations using hydrocortisone pump therapy is achieved without excessive exposure to cortisol. In the three patients we have treated to date, all have attained mean 24 h cortisol concentrations within the normal range and in the case of the two individuals with CAH, mean 24 h 17OHP concentrations were normal (<10 nm) over a 2·5–10-year treatment period compared to an average of 52 nm in children with CAH receiving oral therapy at an average dose of 13 mg/m2 body surface area/day (unpublished observation). The main differences were predominantly due to adequate cortisol replacement between midnight and 06.00 h.
The profiles are extremely reproducible with coefficients of variation of 10%. Dwell time analysis[13]showed that peak cortisol concentrations were similar on pump compared to oral therapy but the time spent with undetectable plasma cortisol concentrations was reduced from 27·0% to 4·4% (P = 0·01).
School attendance increased for all three individuals from 20–30% prepump to 100% for the two people with CAH and to 73% for the boy with Addison's. All three patients have had a marked improvement in their quality of life and now able to participate in sports such as cycling, skiing and surfing. PedsQoL scores have moved to the 80 + level from low 40s.
Conclusions
Cortisol metabolism increases during puberty and often requires a switch to a more frequent regimen of at least four times per day.[14] Simply increasing the dose without altering the frequency will not improve the situation. Hydrocortisone pump therapy is useful in those with rapid clearance of cortisol. This approach can lead to a major improvement in biochemical control of CAH without excessive exposure to cortisol.
In children and young people with adrenal insufficiency when control is not what is expected, it is tempting to invoke poor concordance with therapy. This is often not the case and should not be considered until a careful assessment of cortisol delivery has been undertaken using 24-h profiles and assessment of cortisol bioavailability and clearance. Hydrocortisone pump therapy represents a way in which we can achieve physiological cortisol replacement and manage the adrenal insufficiency. The discussion here highlights pilot study data, which suggest the need for a large national study to identify the role for this approach in adrenal diseases.