Neonatal Life Support
Octubre - 2020
Neonatal Life Support
2020 International Consensus
on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care
Science With Treatment Recommendations
Myra H. Wyckoff, MD, Chair Gary M. Weiner, MD On behalf of the Neonatal Life Support Collaborators
Circulation. 2020;142(suppl 1):S185–S221.
Transition from intrauterine to
extrauterine life at birth requires several critical interdependent
physiological events to occur rapidly to allow successful conversion
from placental to pulmonary gas exchange. 1 Air breathing leads to
significant reductions in pulmonary vascular resistance, which increases
pulmonary blood flow and thereby maintains left ventricular filling and
output (vital for coronary and cerebral perfusion) when the umbilical
cord is clamped.2 When the low-resistance placental circulation is
removed, systemic vascular resistance and blood pressure increase and
right-to-left shunting across the ductus arteriosus decreases.
The majority (approximately 85%) of babies born at term will initiate
breathing within 10 to 30 seconds of birth.3 An additional 10% will do
so in response to stimulation and drying.4 Nevertheless, approximately
5% of term infants receive positive-pressure ventilation (PPV) to
successfully transition, 2% are intubated, 0.1% receive cardiac
compressions, and 0.05% receive compressions with epinephrine.5–8 Although most infants successfully transition without assistance, the
large number of births worldwide means that availability of appropriate,
timely intervention can prevent morbidity and save millions of newborn
lives each year.
Newborn infants who are breathing or crying and have good tone and an
adequate heart rate may undergo delayed cord clamping and should be
dried and placed skin to skin with their mothers to prevent hypothermia.
This does not preclude the need for clinical assessment of the newborn
as secondary apnea, persistent cyanosis, or breathing difficulties can
still occur. For the approximately 5% of newborn infants who do not
initiate adequate respiratory effort after stimulation by drying and
warming, providers must deliver effective ventilation with a face mask.
This is effective in most cases. If it is not effective, providers
should take measures to eliminate mask leaks, check for airway patency,
and ensure that adequate inflation pressures are used; if ventilation is
still not effective, an alternative airway (endotracheal tube or
supraglottic airway) must be considered. Providers must optimize
ventilation because it is the most important step for successful
transition. If, despite efforts to optimize ventilation, the newborn has
a persistent heart rate less than 60/min or asystole, then chest
compressions are needed. Epinephrine and administration of fluids for
circulatory volume expansion may also be required. The neonatal
resuscitation algorithm is shown in Figure 1 and is unchanged from 2015.1,9,10
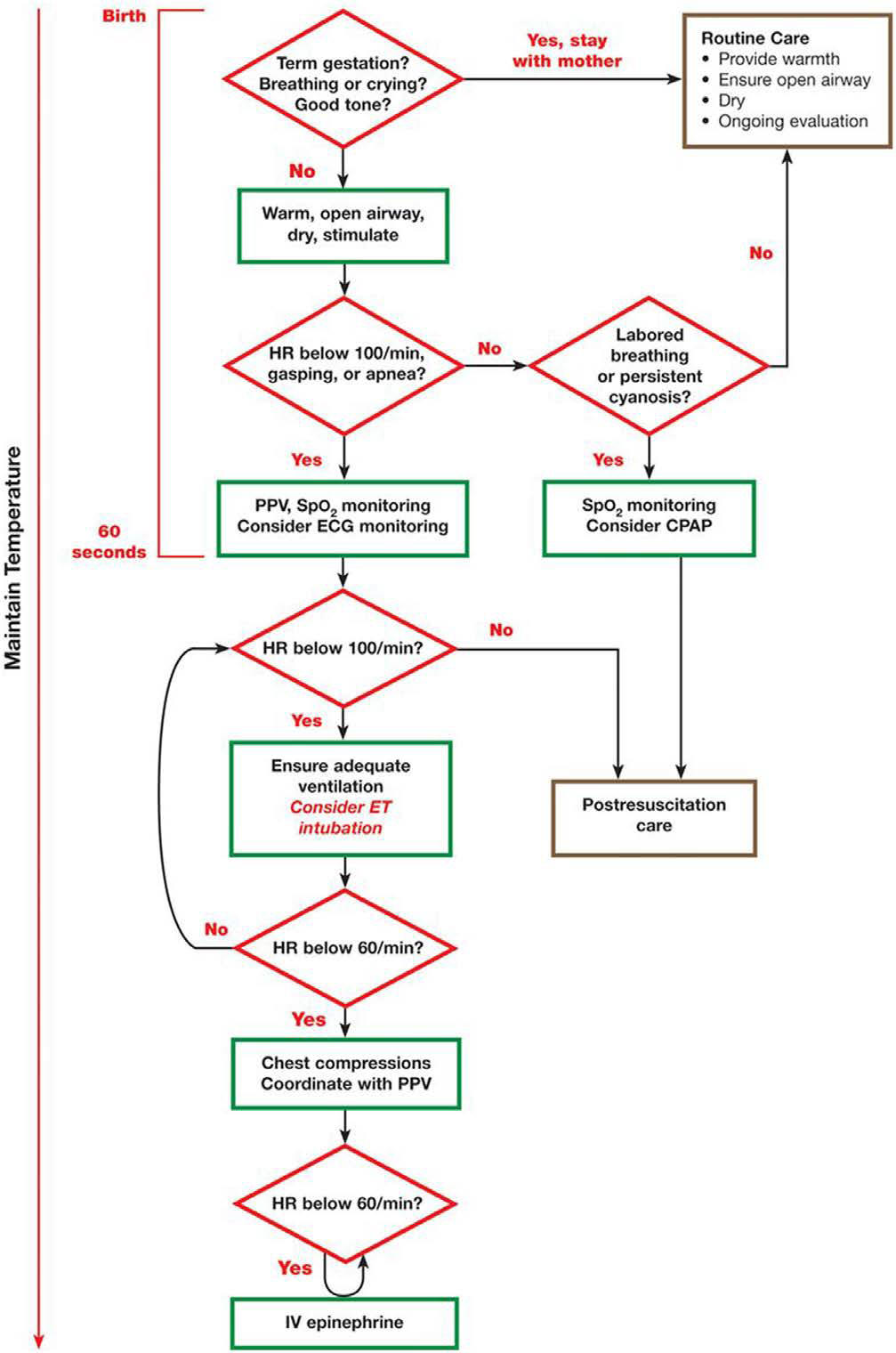
Figure 1. Neonatal Resuscitation
Algorithm.
CPAP indicates continuous positive airway pressure; ECG,
electrocardiographic; ET, endotracheal; HR, heart rate; IV, intravenous;
and PPV, positive-pressure ventilation.
EVIDENCE EVALUATION PROCESS
The 2020 International Consensus on
Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC)
Science With Treatment Recommendations (CoSTR) is the fourth in a series
of annual publications from the International Liaison Committee on
Resuscitation (ILCOR) for neonatal life support (NLS). This 2020 CoSTR
for NLS includes new topics addressed by systematic reviews performed
within the past 12 months. It also includes updates of NLS treatment
recommendations published from 2010 through 2019, based on additional
evidence evaluations.
The 3 types of evidence evaluation supporting this 2020 document are :
the systematic review (SysRev),
the scoping review (ScopRev) y
the evidence update (EvUp).
The choice of the type of evidence
evaluation to perform was determined by consensus of the task force and,
in the case of EvUps, recommendations of ILCOR member resuscitation
councils. The SysRev is a rigorous process following strict methodology
to answer a specific question. The SysRevs informed NLS Task Force
deliberations that are summarized in the NLS Task Force CoSTRs included
in this document. The SysRevs were performed by a knowledge synthesis
unit, an expert systematic reviewer, or by the NLS Task Force, and many
resulted in separately published SysRevs.
1.- Revisión Sistemática (SysRev)
To begin the SysRev, the question to be answered was developed using the PICOST (population, intervention, comparator, outcome, study design, time frame) format. The methodology used to identify the evidence was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA: http://www.prisma-statement.org).
The approach used to evaluate the evidence was based on that
proposed by the Grading of Recommendations, Assessment, Development and
Evaluation (GRADE) working group (https://gdt.gradepro.org/app/handbook/handbook.html).
By using this approach for each of the predefined outcomes, the task
force rated as high, moderate, low, or very low the certainty/confidence
in the estimates of effect of an intervention or assessment across a
body of evidence. Randomized controlled trials (RCTs) generally began
the analysis as high-certainty evidence, and observational studies
generally began the analysis as lowcertainty evidence; examination of
the evidence using the GRADE approach could result in downgrading or
upgrading the certainty of evidence. For additional information, refer
to this supplement’s “Evidence Evaluation Process and Management of
Potential Conflicts of Interest” section.11,11a Disclosure information
for writing group members is listed in Appendix 1. Disclosure
information for peer reviewers is listed in Appendix 2. Draft 2020
CoSTRs for NLS were posted on the ILCOR website (www.ilcor.org) for
public comment between January 15, 2019, and February 20, 2020, with
comments accepted through March 4 for the last NLS CoSTR posted. All of
the NLS draft CoSTRs were viewed a total of 45 032 times, with 279
comments posted.
When online viewing statistics were available for individual CoSTRs,
these are included in the topic information. This summary statement
contains the final wording of the CoSTRs as approved by the ILCOR task
forces and by the ILCOR member councils after review and consideration
of comments posted online in response to the draft CoSTRs. Within this
manuscript, each topic includes the PICOST as well as the CoSTR, an
expanded “Justification and Evidence-to-Decision Framework Highlights”
section, and a list of knowledge gaps requiring future research studies.
In Appendix A in the Supplemental Materials, an evidence-to-decision
table is included for each CoSTR and is based on a new SysRev.
2.- Scoping review (ScopRev)
The second type of evidence evaluation performed to support this 2020 CoSTR for NLS is the ScopRev. ScopRevs are designed to identify the extent, range, and nature of evidence on a topic or a question, and they were performed by topic experts in consultation with the NLS Task Force. The task force analyzed the identified evidence and determined its value and implications for resuscitation practice or research. The rationale for the ScopRev, the summary of evidence, and task force insights are all highlighted in the body of this manuscript.
The most recent treatment recommendations are included. The NLS Task
Force notes whether the ScopRev identified substantive evidence
suggesting the need for a future SysRev to support the development of an
updated CoSTR. Meanwhile, the current treatment recommendation is
reiterated. All ScopRevs are included in their entirety in Appendix B in
the Supplemental Materials.
3.- the evidence update (EvUp).
The third type of evidence evaluation supporting this 2020 CoSTR for NLS is an EvUp. EvUps are generally performed to identify new studies published after the most recent NLS evidence evaluation, typically through use of similar search terms and methodologies used in previous reviews. These EvUps were performed by task force members, collaborating experts, or members of ILCOR member resuscitation council writing groups. The EvUps are cited in the body of this document with a note as to whether the evidence identified suggested the need to consider a SysRev; the existing ILCOR treatment recommendation is reiterated. In this document, no change in ILCOR treatment recommendations resulted from an EvUp. If substantial new evidence was identified, the task force recommended consideration of a SysRev. All draft EvUps are included in Appendix C in the Supplemental Materials.
GENERATION OF TOPICS
After publication of the 2015 International Consensus on CPR and ECC
Science With Treatment Recommendations, 1,9,10 the NLS Task Force,
together with additional neonatal resuscitation content experts (approximately
50 neonatal medicine and nursing professionals, from 17 countries, with
expertise in neonatal resuscitation research, education, and
implementation), reviewed the list of prior neonatal resuscitation
clinical questions to divide them into 3 categories: those that could be
retired, those that remained relevant but required additional clinical
studies to better address the PICOST question, and those with sufficient
evidence to justify a SysRev in the near future. New questions
were also proposed and categorized.
The list was posted for public comment in June 2017, and as a result,
some amendments were made. Using the new ILCOR process of continuous
evidence evaluation (see “Evidence Evaluation Process and Management of
Potential Conflicts of Interest”11 in this supplement), the active
questions were prioritized for SysRevs as ILCOR resources became
available. Other topics were slated for ScopRevs or EvUps as noted above.
The task force met via webinar at least monthly and in person annually;
in addition, the task force met with the larger content expert group
semiannually to present the science and debate and discuss treatment
recommendations. The task force and larger group of content experts
identified and reviewed the published literature and reached consensus
to review the topics included in this manuscript.
2020 TOPICS REVIEWED
I.- Anticipation and Preparation
Prediction of need of respiratory support in the delivery room (NLS 611: EvUp)
Effect of briefing/debriefing following neonatal resuscitation (NLS 1562: ScopRev)
II.- Initial Assessment and Intervention
Warming adjuncts (NLS 599: EvUp)
Suctioning of clear fluid (NLS 596: ScopRev)
Tracheal intubation and suction of nonvigorous meconium-stained newborns (NLS 865: SysRev)
III.- Physiological Monitoring and Feedback Devices
Heart rate monitoring during neonatal resuscitation (NLS 898: EvUp)
IV.- Ventilation and Oxygenation
Sustained inflation (NLS 809: SysRev)
Positive end-expiratory pressure (PEEP) versus no PEEP (NLS 897: EvUp)
Continuous positive airway pressure (CPAP) versus intermittent PPV (NLS 590: EvUp)
T-piece resuscitator versus self-inflating bag for ventilation (NLS 870: ScopRev)
Oxygen for preterm resuscitation (NLS 864: 2019 CoSTR publication)
Oxygen for term resuscitation (NLS 1554: 2019 CoSTR publication)
V.- Circulatory Support
CPR ratios for neonatal resuscitation (NLS 895: EvUp)
2-thumb versus 2-finger compressions for neonatal resuscitation (NLS 605: EvUp)
VI.- Drug and Fluid Administration
Epinephrine (adrenaline) for neonatal resuscitation (NLS 593: SysRev)
Intraosseous versus umbilical vein for emergency access (NLS 616: SysRev)
Volume infusion during neonatal resuscitation (NLS 598: EvUp)
Sodium bicarbonate during neonatal resuscitation (NLS 606: EvUp)
VII.- Prognostication During CPR
Impact of duration of intensive resuscitation (NLS 896: SysRev)
VIII.- Postresuscitation Care
Rewarming of hypothermic newborns (NLS 858: EvUp)
Induced hypothermia in settings with limited resources (NLS 734: EvUp)
Postresuscitation glucose management (NLS 607:EvUp)